The Coutinho-Budd lab is interested in the cellular and molecular aspects of glial cell development and function. Glia are critical components of the nervous system—almost everything that neurons do requires glial support. Significant progress has been made in understanding neuron-glia interactions at synapses and axons, but how glia support neuronal cell bodies remains poorly defined.
Many glial cell types in the mammalian nervous system have subsets that interact with neuronal cell bodies (e.g. protoplasmic astrocytes, satellite microglia, and perineuronal oligodendrocytes in the brain and spinal cord, Müller glia in the retina, and satellite glia in peripheral ganglia). Unfortunately, the genetic tools needed to study glial interactions only at neuronal cell bodies don’t exist in mammals.
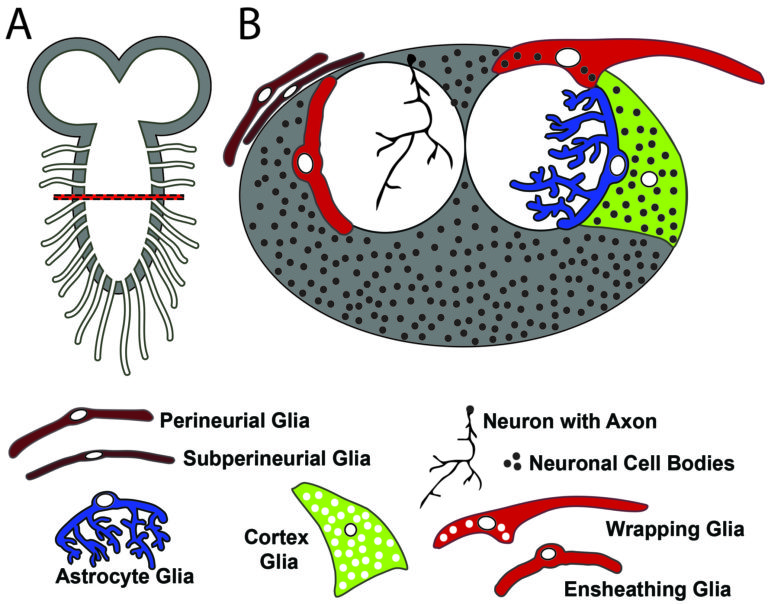
My lab takes advantage of Drosophila cortex glia—a remarkable subclass of glia that extend fine processes to individually wrap almost every neuronal cell body in the central nervous system—as a model to study these neuron-glia interactions in vivo. Cortex glia are thought to secrete factors for neuronal development, buffer ions and nutrients in the extracellular environment, and remove debris during development and after injury. Animals with dysfunctional cortex glia exhibit behavioral deficits ranging from poor coordination and impaired locomotion to seizures, and a lack of cortex glia can be lethal, yet these cells have not been extensively studied. My lab aims to better understand how cortex glia develop, communicate with surrounding neurons and glia, and contribute to overall nervous system function.
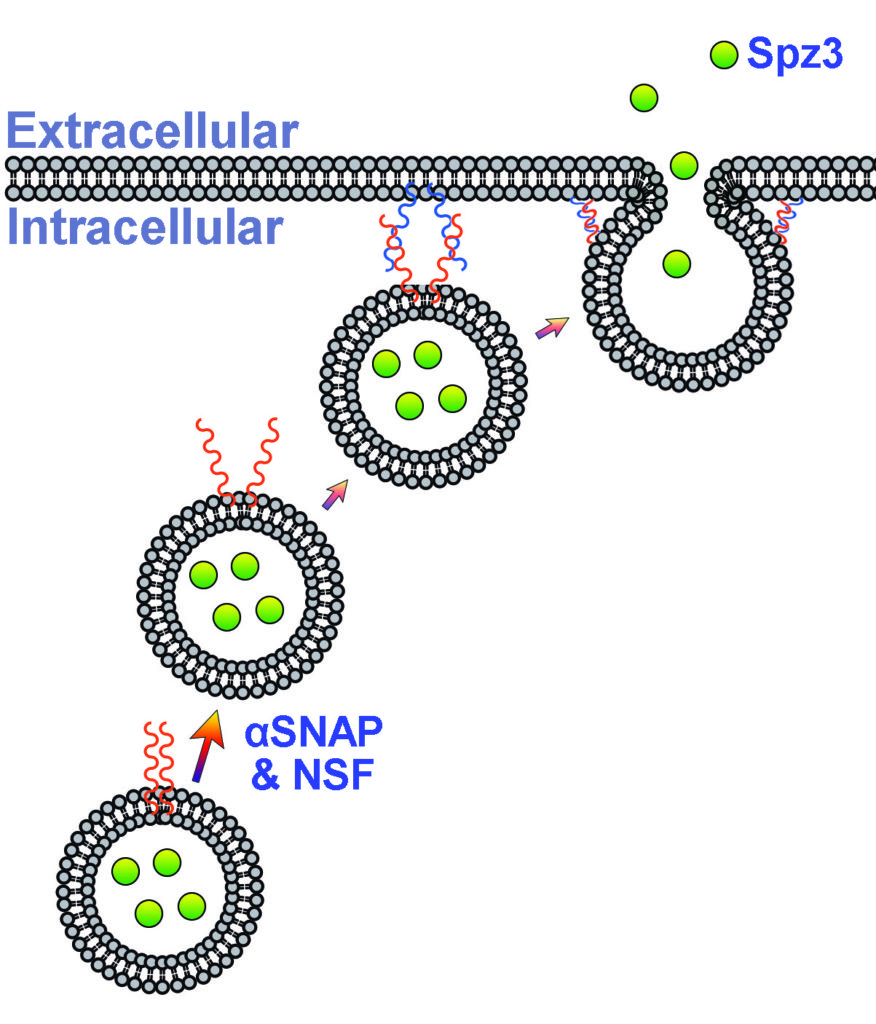
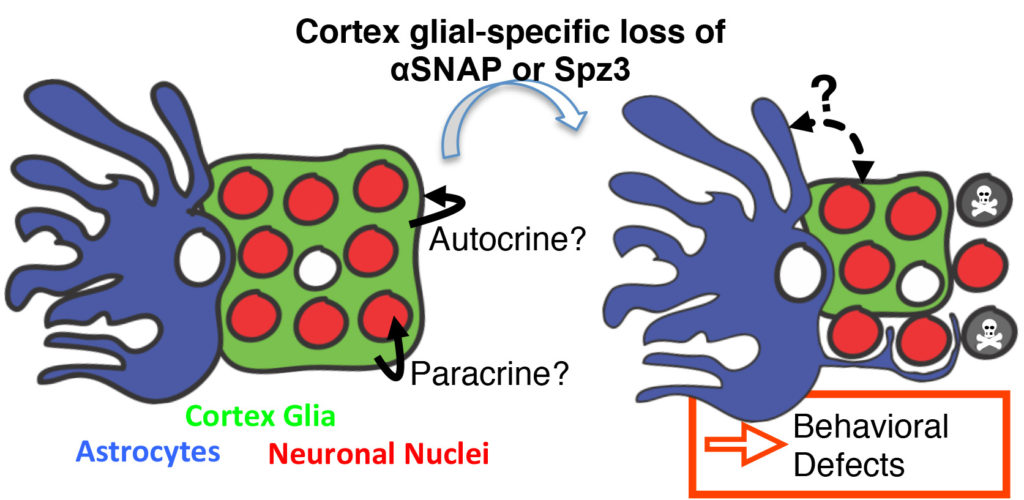
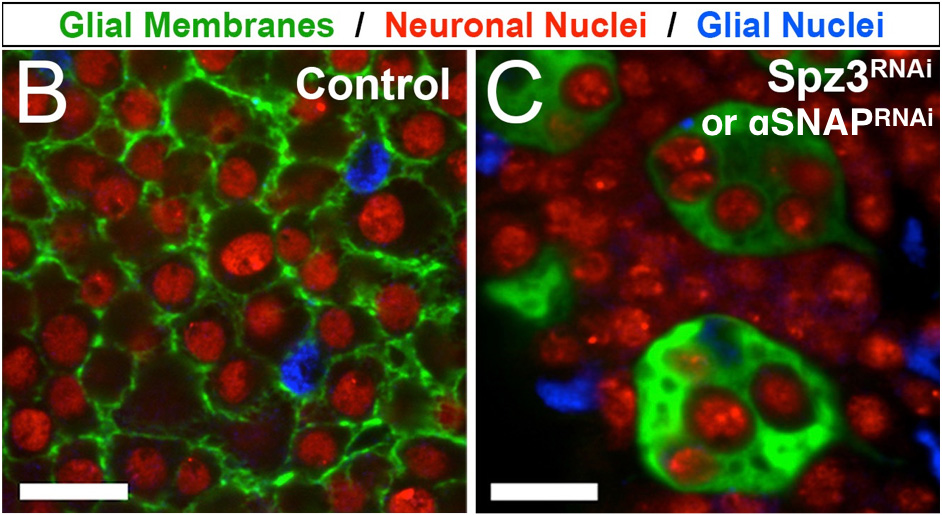
One approach in understanding how neurons and glia communicate is to use biosensors to visualize molecules in real time in vivo (i.e. living brains). Here, a calcium indicator expressed in cortex glia (green) shows calcium signaling events occurring in the fine processes that surround neuronal cell bodies (red).